Murray Lab (2016-2022)
The Murray Lab, established at the Sainsbury Wellcome Centre in 2016, focused on the neural circuits that underlie movement, specifically the vestibular system in order to understand how the brain uses sensory information to modify ongoing motor actions. Previously Group Leader at SWC, Dr Andy Murray is now CEO and Co-Founder of Sania Therapeutics, developing novel gene therapies to treat neurological disorders.
Research Area
The Problem
The brain has only one means of interacting with the world – by generating motor commands to control muscles. Movements come in many forms, but all movements must be rapidly adaptable and modifiable in response to changes in in the body and the surrounding environment.
Consider the complex set of reflexes you would make if you were to slip as you walk down some steps. Within hundreds of milliseconds your brain can interrupt your ongoing movement, shift the position of your legs and generate a command to grab a nearby handrail. Or, think about how we maintain our balance while standing on a moving train. Without any conscious effort the brain constantly tweaks the activation of hundreds of muscle groups, shifting the position of the body to counteract the train’s movement.
Correct implementation of these motor commands requires the brain to maintain an up to date representation of the body’s position and motion in space, a detailed awareness of the surroundings (such as, there is a handrail to my left) as well as the impact that the surrounding environment has on the body. These are the type of neural computations upon which the Murray lab is focussed.
Our Research Approach
Information about both the current state of the body, as well as the surrounding environment, is communicated to the brain through various sensory modalities.
Our lab’s focus is on the vestibular system, a sensory system that provides information about rotation and acceleration of the head and which is critical for our ability to move, balance and navigate.
The vestibular system presents us with a unique experimental opportunity where we have complete control over sensory information entering the nervous system, combined with the ability to precisely measure the system’s output, or motor commands. Through study of the brainstem and the spinal circuits that coordinate vestibulo-motor behaviours we strive to obtain important insights into sensory-motor transformations throughout the nervous system.
Research Topics
1. Contextual adaptation of sensory-motor processing
How we perceive and respond to a sensory stimulus depends heavily on the environmental context. For example, the sensory-motor reflexes that maintain our balance are generally more prominent when our balance is threatened. How does the brain interpret one particular environment as being threatening, and how does it then use this information to adapt sensory-motor reflexes?
The lateral vestibular nucleus (LVN) can generate postural reflexes that maintain balance. Two complimentary approaches are employed in our study of how LVN circuit dynamics are altered in different environmental contexts. First, we are examining the input circuitry to the LVN in order to determine which circuits could influence reflex gain. Second, we are developing mouse behavioural approaches that allow us to tune the level of balance threat. This complementary circuit and behavioural approach should help us to understand how the brain can control the gain of sensory-motor reflexes in different environments.
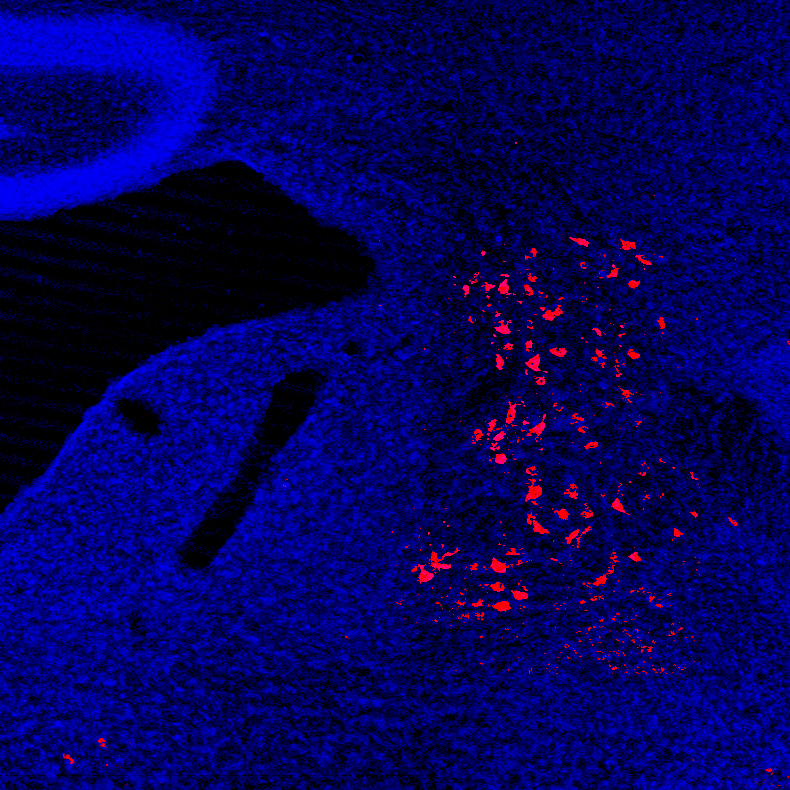
Neurons in the mouse lateral vestibular nucleus labelled by fluorogold injection in the spinal cord.
2. Adapting to our own motor actions
Whether running for the bus, reaching for a book or stepping onto an escalator most of the movements we make present two problems for the brain:
i) Limb and body movements can disrupt our balance, so how does the brain ensure our balance is stable when the body is in motion?
ii) Self-generated limb and body movements activate sensory receptors in the same way as externally applied perturbations. How does the brain distinguish between these two types of sensory input?
The nervous system solves these seemingly separate problems in the same way, by constantly predicting the consequences of its own actions and making according adjustments to sensory-motor.
Preparing for movement through anticipatory postural adjustments
Every limb and body movement has the potential to destabilise the body. The simple act of taking a step, or reaching for an object can move the body’s weight too far from the base of support leading to a fall. To prevent this, the nervous system predicts the consequence of a movement before it is performed and generates an anticipatory postural adjustment (APA) – a pre-emptive adjustment of trunk and limb muscles in anticipation of the upcoming movement. In the lab we study how APAs are generated in order to probe how the brain predicts the consequences of its own movements.
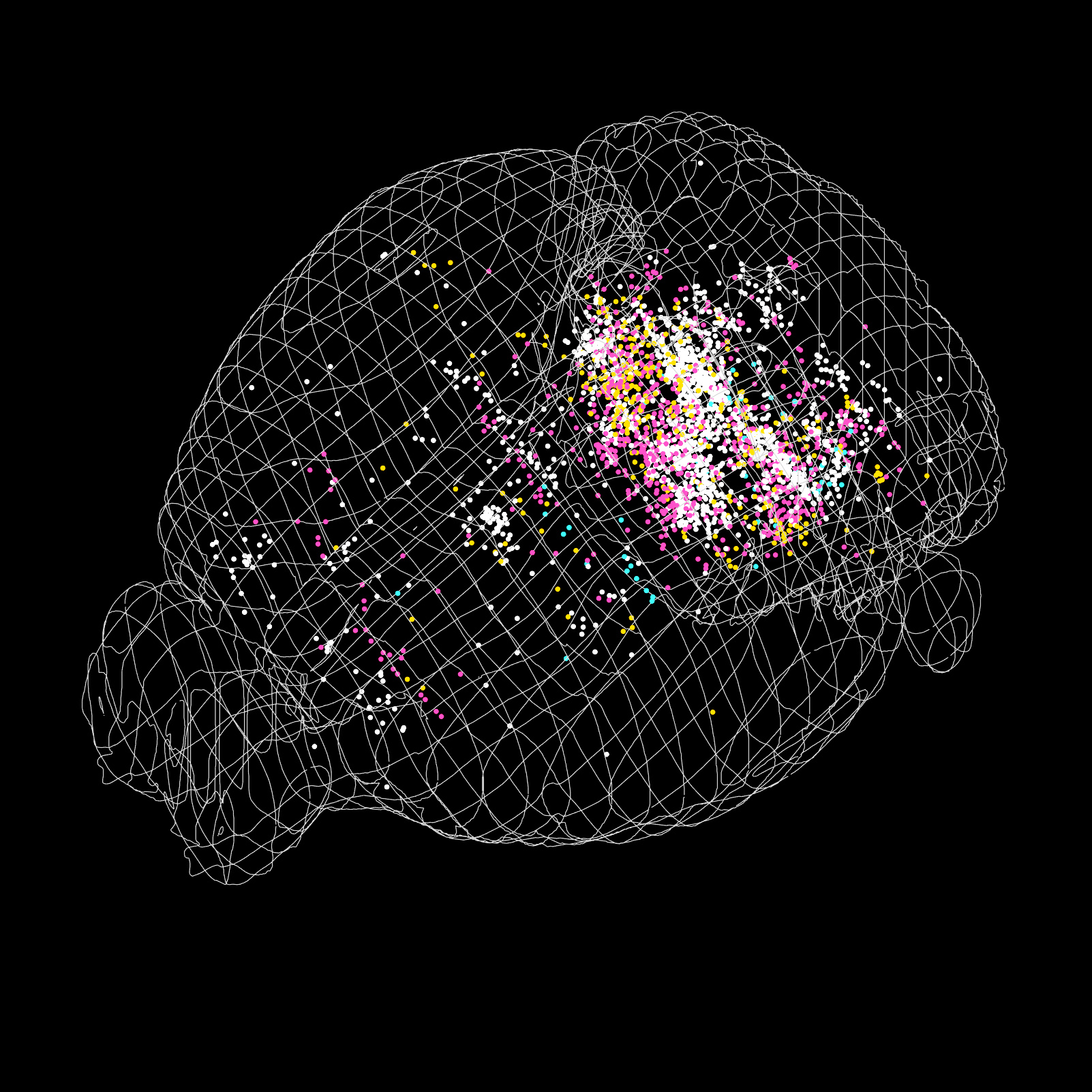
Neurons in the mouse brain that directly innervate postural circuits, identified using monosynaptic rabies tracing. (Neurons are shown on the Allen reference atlas using software developed by Nick Steinmetz & Philip Shamash in the laboratory of Kenneth Harris and Matteo Carandini at UCL.). Egzona Morina, Murray Lab
Tuning of vestibular processing during movement
Sensory input from the vestibular system provides information about acceleration and rotation of the head. However, the brain can alter this sensory inflow through the actions of the efferent vestibular system (EVS), a group of brainstem neurons that innervate the vestibular periphery and can alter firing in hair cells and vestibular afferents. One hypothesis regarding the function of the EVS is that these neurons receive a copy of motor output and use this information to tune the vestibular system accordingly. In the lab, we are using a combination of mouse genetics and viral approaches to target the EVS and understand its role in distinguishing self-generated and externally imposed motion.
3. Neurotechnology - virology
Central to our goal of understanding vestibular-motor behaviour is the availability of tools for the targeting, manipulation and mapping of neural circuits. Our lab, therefore, also develops new viral technologies that allow us to map the connections between neurons as well as monitor and manipulate their activity. It is our hope that the technology developed in our lab will aid both in our goal of understanding sensory-motor circuits, as well as being valuable to the wider neuroscience community.
Selected Publications
Balance Control Mediated by Vestibular Circuits Directing Limb Extension or Antagonist Muscle Co-activation
Murray AJ, Croce K, Belton T, Akay T, Jessell TM
Cell Reports (22(5): 1325-1338) (doi: 10.1016/j.celrep.2018.01.009)
30 January 2018
Reviewing the Role of the Efferent Vestibular System in Motor and Vestibular Circuits
Mathews MA, Camp AJ, Murray AJ
Frontiers in Physiology (8: 552) (doi: 10.3389/fphys.2017.00552)
02 August 2017
Rabies Virus CVS-N2cΔG Strain Enhances Retrograde Synaptic Transfer and Neuronal Viability
Reardon TR, Murray AJ, Turi GF, Wirblich C, Croce KR, Schnell MJ, Jessell TM, Losonczy A
Neuron (89(4): 711-24) (doi: 10.1016/j.neuron.2016.01.004)
17 February 2016
Mapping Sensory Circuits by Anterograde Transsynaptic Transfer of Recombinant Rabies Virus
Zampieri N, Jessell TM, Murray AJ
Neuron (81: 766-778) (doi: 10.1016/j.neuron.2013.12.033)
19 February 2014
Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory
Murray AJ, Sauer JF, Riedel G, McClure C, Ansel L, Cheyne L, Bartos M, Wisden W, Wulff P
Nature Neuroscience (14: 297–299) (doi: 10.1038/nn.2751)
30 January 2011