Cuttlefish camouflage: understanding the brain mechanisms
An interview with Dr Dominic Evans, Max Planck Institute for Brain Research (Frankfurt am Main), conducted by April Cashin-Garbutt
Cuttlefish are masters of disguise, capable of transforming their appearance to blend seamlessly into their surroundings. This remarkable ability to camouflage is both visually and scientifically striking and scientists are now starting to reveal the complex neural mechanisms at play.
In a recent SWC seminar, Dr Dominic Evans, a former student at the Sainsbury Wellcome Centre and now at the Max Planck Institute for Brain Research in the group of Prof. Gilles Laurent, discussed his work on the circuits and physiology of the key brain region – the optic lobe – that performs this behaviour in the common cuttle fish (Sepia officinalis).
In this interview, Dr Evans shares some of the challenges of studying these exotic creatures and the broader implications for discovering general principles of how brains drive behaviour.
What first inspired you to study the camouflage mechanisms of cephalopods like cuttlefish and octopus?
I’ve long been interested in how animals across the animal kingdom exhibit complex and remarkable natural behaviours. The camouflage of cephalopods is particularly striking, as it’s directly controlled by the brain: motor neurons are switching thousands of skin pixels on or off using muscle contractions.
These animals are showing us how they perceive the world on their own skin, which is fascinating and very powerful experimentally, as normally we do not know what animals are thinking. They have very few ways to tell us, but the cuttlefish have these complex patterns. The whole camouflage system is just a really unique opportunity to study how vision and complex behaviour work.
With the advent of new computer vision techniques and AI, harnessed by Prof. Laurent’s lab (Reiter et al., 2018), we can now record all the skin pixels in great detail, which means we can quantify the whole behaviour of these animals at the level of motor output.

How much is known about the way cephalopods transform visual scene information into full-body camouflage patterns? Which brain regions allow them to perform this remarkable behaviour?
The first step is light coming into the eye, and in fact they’ve evolved – independently – a high-resolution camera-type eye that’s not unlike our own.
Cephalopods are highly visual, and they devote over 70% of their whole brain volume to their optic lobes, which process visual input from the eye in a way that allows them to produce new textures on their skin.
We know the major anatomical pathways involved, thanks to work done largely at UCL over 50 years ago by JZ Young, Brian Boycott and colleagues.
In the 90s, Young worked with Marion Nixon at the Wellcome Trust to assemble a huge collection of this anatomical work, including beautiful morphologies of neurons from different cephalopods, to guide future research (The Brains and Lives of Cephalopods, OUP).
A small field of researchers, including at Marine Biological labs in Plymouth, Naples and Massachusetts have worked on topics like cephalopod behaviour and early vision. However, we only roughly understand the function of brain regions and pathways, and are only now finding out how neurons are connected up in circuits.
How are you exploring the neural circuits involved?
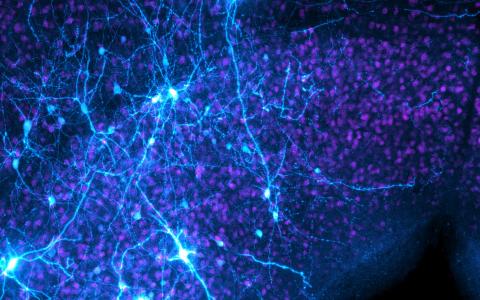
We're using several approaches to build a comprehensive picture of the system. This includes connectomics, which involves reconstructing the circuitry of the visual system at the synaptic level using electron microscopy (EM). Together with my colleague Ali Karimi, and with Christopher Bleck and Michael Reiser at HHMI Janelia Research Campus, we have acquired volume EM datasets of the optic lobe and are identifying the microcircuits of early vision: the equivalent of complex retinal circuits in vertebrates.
We’re simultaneously trying to understand its function using high-density neural activity recordings while animals are free to swim around and behave.
Brian Boycott recounted that this was the dream experiment of the UCL team in the 1960s, and technical limitations made it impossible for them to pursue such functional studies further. A fellow postdoc, Margot Elmaleh, and I are very excited that we are now able to do it, and gain a functional understanding of neural camouflage mechanisms.
What are the main challenges you face when studying cuttlefish?
Although we study the common cuttlefish, Sepia officinalis, they are still quite exotic as a lab animal, but animal care staff and vets at the MPI Brain Research have made some important leaps regarding husbandry, including breeding them over multiple generations in the lab.
When they hatch, they are about 1cm and grow throughout their lives, requiring a set of tanks of different sizes as they can grow up to 30cm in size by the end of their natural lifespan of up to 2 years. A colony therefore needs thousands of litres of seawater to thrive in the lab.
What have been your key findings so far?
Camouflage in cephalopods is even more complex than we thought! The animals never produce the same pattern twice, and they slowly update and improve the pattern match when presented with a new environment (Woo*, Liang* Evans et al., 2023). They’re not constantly showing a camouflage pattern and trying to match the background; they can also show other patterns to scare off predators or social patterns to other animals. We found that the scary pattern likely has a different control network.
Our ongoing connectomics work is showing that even at the early stages of visual processing, there is some convergence with vertebrate vision.
We've also been able to make neural recordings in freely-moving animals while they camouflage.
Body pattern behaviour and skin pixel dynamics in response to approaching visual stimuli
Top: While engaged in background-matching camouflage, animals are presented with approaching motion stimuli (5x playback speed).
Bottom left: This causes them to break camouflage from ‘mottle (M)’ or ‘disruptive (D)’-like start (s) patterns, and display a brief ‘blanching (B)’ pattern by contracting almost all chromatophores (skin-pixels), in a diematic display thought to be startling or threatening to approaching predators.
Afterwards, the animal usually ends up (e) back at a similar camouflage pattern (blue, green trajectories in chromatophore space), but sometimes a different camouflage pattern is reached (red).
Bottom right: To restore camouflage, the motor system is constrained to pass through a ‘reset sequence’ where different groups of co-active skin pixels (yellow, red, green etc) are recruited in a stereotyped order. Adapted from Woo*, Liang* et al 2023.
How does the optic lobe of Sepia compare to other species, including vertebrates?
Three types of cephalopods can control skin pixels with vision – squid, octopus, and cuttlefish. They are the only animals with an optic lobe that has a multi-layer cortex wrapped around a tree-like structure. This multi-cellular neural tree is not present in other invertebrates, and the structure is much simpler in another cephalopod called nautilus, which doesn't have skin pixels for camouflage.
The evolution of this highly specialised and unique optic lobe might well be related to their feats of camouflage, turning visual textures that they see into similar textures they can produce on their skin.
Were you surprised by any of your results?
Yes, the animals are even more fascinating than we imagined! The closer we look, the more surprising features we find. Cephalopods are proving to be a useful model for modern systems neuroscience, as Young and colleagues hoped for. While laboratory mice are incredibly useful, there are many behaviours they cannot do, and thus some neural mechanisms are not there waiting to be discovered.
Recent years have shown a re-found appreciation for the benefits of a neuroethological approach in research, meaning studying animals who are specialists at particular natural behaviours, even if they are not established model systems. Monumental advances in molecular biology mean that important genomic and transcriptomic information is not just the preserve of mice and flies anymore.
What are the main implications of this research?
Because we’re studying a visual system specialised for a behaviour that we can’t do, when we find mechanisms of computation that are nevertheless common to ours, we hope we have found a general principle of how vision and brains work. This research could be therefore be useful for understanding how biological neural networks compute, and process images in general, with implications for computer vision and AI.
What is the next piece of the puzzle you hope to solve?
I’m aiming to have an integrative view of how cephalopods make decisions moment to moment and how they choose to engage in camouflage and other behaviours. We also want to use their patterns as readouts for other internal states.

About Dominic Evans
Dominic Evans is a postdoc at the Max Planck Institute for Brain Research (Frankfurt am Main, Germany), studying the neural basis of camouflage behaviour in the department of Prof. Gilles Laurent. After studying Neuroscience at UCL, Dominic joined Prof. Tiago Branco’s lab at MRC Laboratory of Molecular Biology in Cambridge and later at UCL Sainsbury Wellcome Centre, working on escape behaviour in mice.
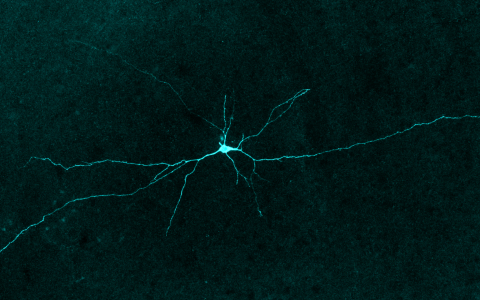
Banner image credit: Stephan Junek, MPIBR
*These authors contributed equally.