
Motivated mice matter: Refining fluid control
By Hyewon Kim
“Happy animals equals good science” remarked Lillianne Teachen, Chair of the SWC/Gatsby 3Rs Group and Senior Research Technician in the Akrami Lab, who also chaired a workshop held at SWC last month on refining the use of fluid control in rodents. “We can’t replace fluid control, but we can make it better and more reproducible – refine the controls and standardise the methodology to be used across various laboratories that have similar scientific needs.”
In neuroscience labs around the world, researchers go about understanding complex behaviours like decision-making, risk-taking, or learning with the help of model organisms. Mice and rats can be trained on behavioural tasks with various rewards. When positive reinforcement fails and under careful expert supervision, rodents are sometimes temporarily offered fluids in a controlled manner in order for their training to be successful.
How can fluid control be made better for both animals and the science? The SWC/Gatsby 3Rs Group teamed up with the NC3Rs to run a half-day workshop at SWC, bringing together experienced researchers and experts from across the UK.
Giving animals free access to citric acid water
Nate Miska, Senior Research Fellow in the Mrsic-Flogel Lab, presented in the workshop a relatively novel way to use citric acid (CA) for keeping mice motivated. A previous study from the International Brain Laboratory (IBL) had shown that up to 4% CA mixed in the water, thanks to its slightly sour taste, can work as a good substitute for water control in mice performing a decision-making task while maintaining healthy weights.
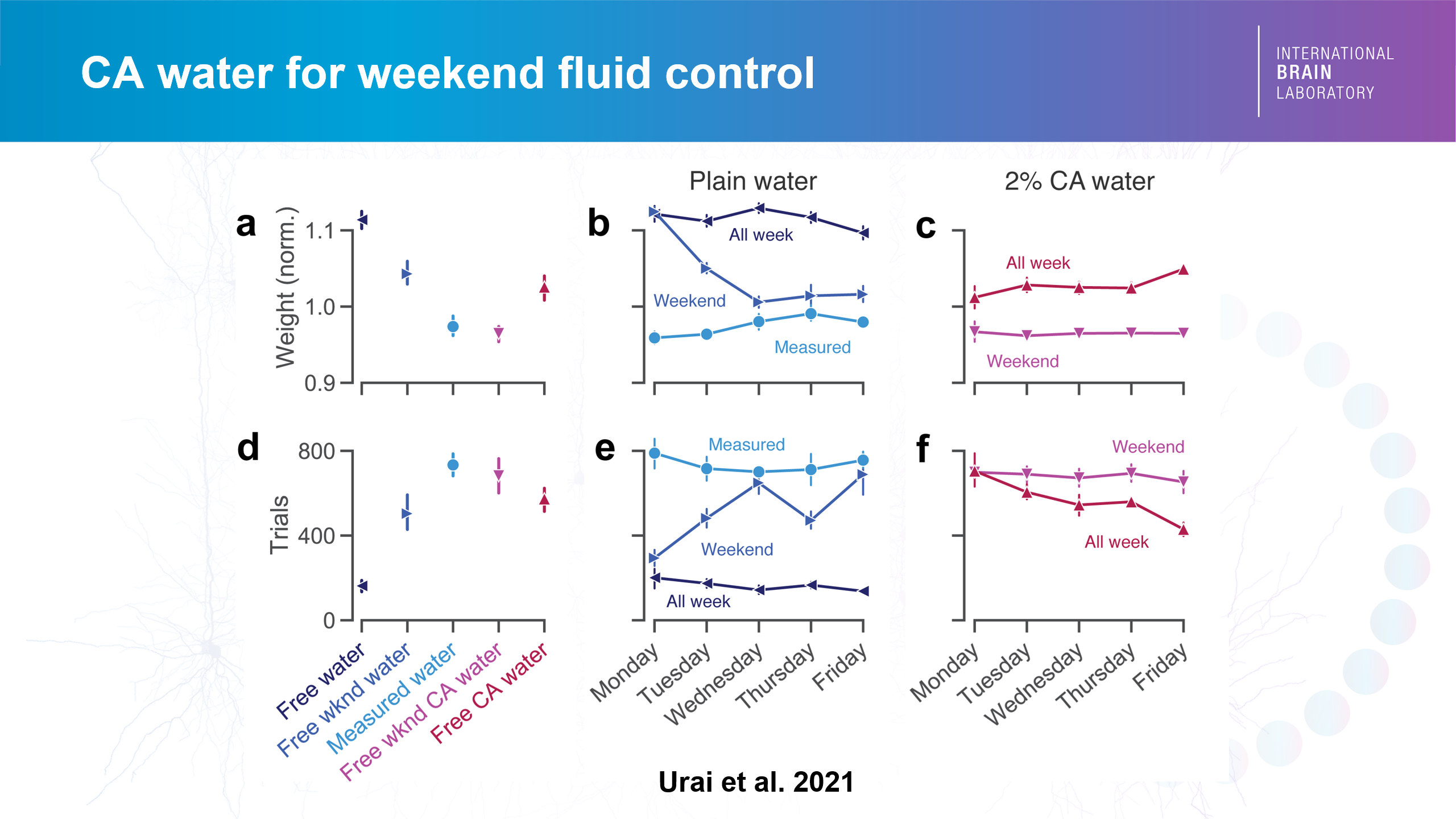
Credit: Urai et al. 2021
The CA method allows the mice to have free access to the water instead of having it weighed out individually, which addresses the individual needs of each mouse. The mild aversiveness of CA-mixed water means the mice stay fluid-controlled. The CA method is also better for the animals themselves. “Not all mice need the same amount of fluid. So weight is not always the best measure to make sense of their motivation levels,” Nate explained.
Implementing the CA water method in his own experimental mice at SWC for the past three years with no observed negative consequences, and monitoring the change in their body weight, Nate concluded that ad lib CA water can indeed replace manually measured water. He also suggested that CA water could be used to ease animals into a fluid control regime, as well as to supplement fluids in animals during training when animals may not receive sufficient water from the task itself.
How do you know if a particular fluid control method is working? First and foremost, the animal needs to be healthy. A researcher monitors the weight of the rodent as well as its general health condition and also assesses its behaviour i.e., sees if the animal is doing the task across multiple trials in the experiment. “There is a noticeable difference in the number of trials completed between less or more fluid-controlled mice,” Nate said.
Gaining insight from experts on fluid control
Another researcher from the IBL gave a talk focused on making reproducible recordings of water control using the IBL system. The comprehensive recording practice, tried and true, would be similar for each animal and key for reproducibility.
The workshop also brought to attention an automated water administration system called Water R. The cage rack in which rodents are housed would implement a smart, hands-free method to give differing amounts of water to the animals depending on welfare and experimental needs.
In another talk, a researcher from Imperial College London shared the parameters they used for controlling fluids in mice, showing that in some experiments there was more control than in others. “It was useful to see how different people from different institutions go about doing water restriction,” Nate shared.
Driven by a collective need
“The use of fluid control is broadening across the neurosciences. It is possible to improve the quality of the data obtained while still prioritising the welfare of the animals used in scientific studies,” commented the NC3Rs organisers Stephen Turnock, Chris Barkus, and Laura McKillop. “These methodology-focused events are an important part of facilitating the application of the 3Rs at a local level. Bringing people together to reflect on best practice and hear about relevant refinements ensures advances made by individuals are disseminated, maximising the 3Rs impact they have.”
For both Lillianne and Nate, it is the first time that they have attended such a specific and focused workshop on refining fluid control. “Compared to more general workshops or conference, this discussion was right up my alley and kept me thinking about the different methods,” Lillianne said. “The workshop was very well organised and each talk was interesting in its own right.” Nate shared that he had attended seminars focused on scientific topics or standardisation techniques, but this was the first time he’d participated in discussions around such a specific 3Rs topic.
Future workshops on more 3Rs methods are welcome at SWC. Topics in discussion involve refining head fixation, such as tethering neural recordings in rats, and a more general control workshop that is not just limited to fluids, but any methods to keep mice motivated for a task. These include ways to replace fluid control altogether or more significant and all-encompassing refinements, such as direct stimulation of the ventral tegmental area dopamine neurons using an embedded electrode.


