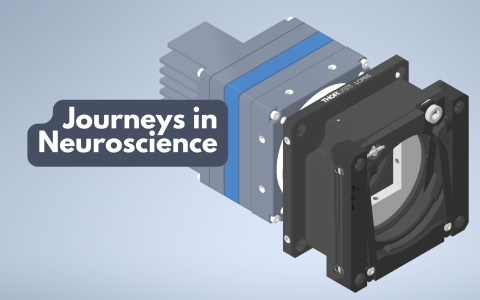
Mini microscopes: advances in two-photon imaging for neuroscience
An interview with Dr Weijian Zong, Kavli Institute for Systems Neuroscience, Norwegian University of Science and Technology, conducted by April Cashin-Garbutt
To understand complex cognitive functions, researchers need tools capable of capturing the activity of large populations of neurons with high spatial resolution. While traditional extracellular recording techniques have enabled the monitoring of hundreds of neurons simultaneously, they fall short in revealing the morphology of neural circuits.
In a recent SWC Seminar, Dr Weijian Zong, Group Leader at the Kavli Institute for Systems Neuroscience, presented his work developing new generations of miniature two-photon (2P) microscopes. These miniscopes offer resolution, field of view, speed, and z-scanning capability similar to that of 2P benchtop microscopes. In this interview, Dr Zong discusses the impact of these advances on neuroscience research.
What are the main drawbacks of traditional extracellular recording techniques for studying brain microcircuits?
Traditional extracellular recordings, like those using silicon probes, allow us to record from a hundred to a thousand neurons simultaneously. However, they lack spatial resolution and morphological context.
You can't see the neurons you're recording from, which makes it difficult to study how individual synapses or dendrites integrate information. Plus, long-term tracking is challenging because the brain shifts slightly over time, making it hard to record from the same neurons across days or weeks.
What benefits does 2P functional imaging offer?
2P imaging lets you see the neurons you're recording from, down to subcellular structures like dendritric spines. This spatial resolution is crucial for understanding how single neurons compute information.
This morphology information also enables long-term tracking of the same neurons, which is essential for studying processes like learning, memory, and aging. Additionally, by using genetically encoded indicators, we can identify different types of neurons and study their specific roles in neural computation.
Why are conventional 2P imaging systems so bulky?
It’s not that they had to be bulky – it’s more that there needs to be someone with motivation or resources to make them smaller. These systems are complex, involving lasers, optics, scanners, detectors, and more. No single company builds all the components from scratch, so systems are often developed by bringing together parts from multiple companies. And so, there was a big challenge for a single group to make everything smaller.
But now, thanks to advances in consumer electronics, like smartphone camera lenses and self-driving car scanners, we can access high-performance, miniaturised components that make compact systems possible.

How much progress has been made over the last two decades in developing portable mini 2P microscopes?
The field has progressed from early prototypes weighing 25 grams to today’s systems that rival benchtop microscopes in resolution and sensitivity.
Initially, components like objectives, scanners, and fibres weren’t optimised, and indicators weren’t sensitive enough. But with the development of better calcium indicators like GCaMP6 and improved components, we’ve entered an “explosion phase” where many labs and companies are pushing the technology forward.
What can today’s miniaturised 2P microscopes do?
We are almost reaching the same imaging resolution as the normal 2P microscopes. We can do sub-micron resolution, visualise dendrites and spines, and image hundreds of neurons with near single-cell resolution. Detection efficiency is now on par with or even better than benchtop systems.
Some systems can even perform multi-wavelength excitation and volumetric imaging in the same time as benchtop. It is also possible to do three-photon microscopy with miniscopes.
There’s still room for improvement in imaging speed and advanced features like adaptive optics or holographic stimulation, but we’re getting close to matching benchtop capabilities.
What are some of the key research questions that these new 2P microscopes have enabled your group and your collaborators to study?
They’re especially useful for studying complex behaviours like spatial navigation, where animals need to move freely. They also allow us to combine functional imaging with genetic and transcriptomic data, helping us link neural activity to gene expression.
This opens up new possibilities for studying social behaviour, and behaviours that are sensitive to stress level such as sleep – areas where traditional head-fixed setups fall short.
What advice would you give to labs interested in adopting this technology for their own research?
First of all, ask yourself whether freely moving two-photon imaging is essential for your research. It’s a powerful tool, but adopting new technology takes time and resources. If you’re committed, it helps to have someone in the lab with a background in optics or engineering – or at least someone passionate about learning.
Start by building a system from scratch using open-source resources, or consider purchasing a commercial system for short-term goals. And above all, invest in good surgical skills – successful imaging depends heavily on high-quality preparations.
What are the next steps and what do you think the future holds for 2P microscopy?
From a scientific perspective, we need some key scientific questions that can only be answered using this technology – something that really justifies its value. Technologically, there’s still room to grow in areas like adaptive optics and holographic stimulation.
What advice would you give to emerging scientists interested in developing the cutting-edge imaging techniques of the future?
Do it because you love it – not to publish a big paper or make money! You should be driven by the joy of creating something new and solving problems no one else has tackled. You have to be innovative and think outside the box. Don’t try to compete with industry on speed or scale – focus on ideas that only you can bring to life.

About Dr Weijian Zong
Weijian Zong is currently an associate professor, and the group leader of the neurophotonics lab at the Kavli Institute for Systems Neuroscience, Norwegian University of Science and Technology in Trondheim, Norway. He had his post.doc training in the lab of Prof. Edvard Moser and Prof. May-Britt Moser. He holds a BSc in Electrical Engineering from Peking University and a Ph.D. in biophotonics, supervised by professors Heping Cheng and Ming Fan.
Dr Zong's research is focused on developing cutting-edge imaging techniques to understand brain function and behaviour. His research has contributed significantly to the development of miniature two-photon microscopy for brain imaging in freely behaving mice.
He holds several invention patents in optical and imaging and has received numerous fellowships and awards for his work, including the Tycho Jæger’s Prize in Electro-Optics in 2022, the Marie Skłodowska-Curie Individual Fellowship in 2019, China's top 10 scientific achievements and Shitsan Pai Award for Young Biophysicists Award in 2017.