Targeting neuronal cell types for repair
An interview with Professor Botond Roska, Institute of Molecular and Clinical Ophthalmology (Basel), conducted by Hyewon Kim
How does understanding the visual system and applying that understanding to treat its disorders come hand-in-hand in a research lab?
Professor Botond Roska recently gave a SWC seminar on neuronal cell-type based medicine for visual dysfunction repair. In this Q&A, he shares the historical background that paved the way for the type of gene therapy his group aims to develop, how he got into studying vision, key findings, and more.
What was already known about visual dysfunction repair?
The history goes back to a lot of heroic experiments done almost 100 years ago with electrodes in the brain to produce phosphenes. More recently, it was Mark Humayun who put electrodes in the retina and showed that with stimulation you get phosphenes – you see a spot. And then, there was a lot of development of electrode arrays and the advent of gene therapy.
Optogenetic therapy is the most modern version of neurostimulation where you don’t need surgery to put in an array. Basically, you put your sensor into the cell using gene therapy, that is a single injection to the eye, and have all the electronics outside.
There is a lot of known about visual dysfunction, of course. This is a part of medicine called ophthalmology. In the last decades we learned a lot about the genes that cause inherited monogenic retinal diseases and about the retinal cell types that are affected in these diseases. However, what are the causes of the most prevalent diseases, which are not monogenic, such as macular degeneration and glaucoma, are today the major unanswered questions.
What got you interested in researching this topic?
I came to study vision completely randomly. I originally studied medicine and mathematics. When I finished my medical studies, what was clear to me was that I didn’t want to be a practicing medical doctor. I didn’t know what to do. One day, I had dinner with someone who studied the retina, Frank Werblin, and I realised the topic was very exciting to me. Eventually, this led me to pursue a PhD at Berkeley. And from there on, I became very interested in vision. At one point, it occurred to me that I can combine this understanding with my medical background to design therapies.
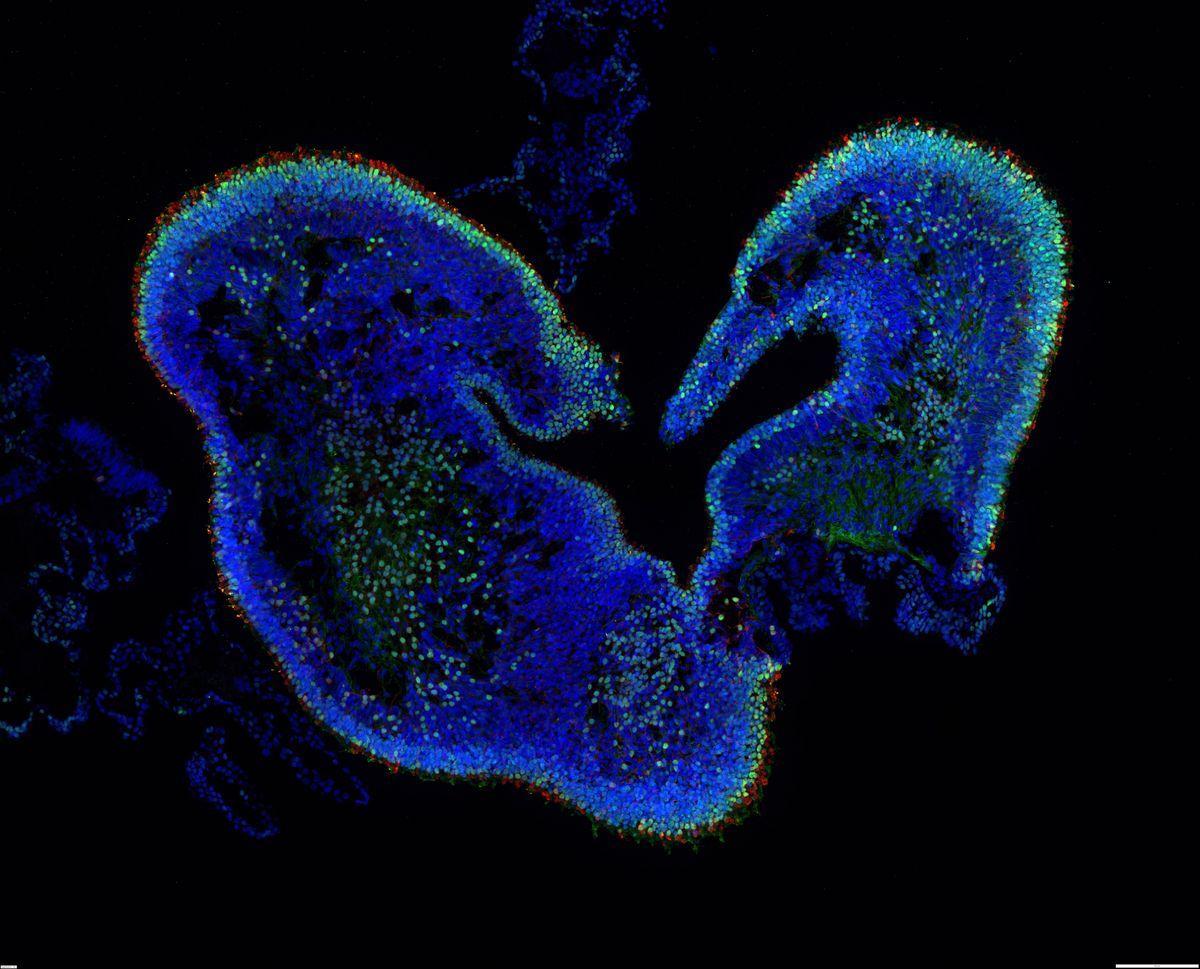
Retinal organoid, thin section. Credit: IOB.ch
How does your research try to reveal the structure and function of visual circuits as well as understand disease mechanisms?
We combine different technologies because understanding requires not a single technology but a combination of them. Physiological technologies are key to understanding how the retina or visual system computes information. For that, we use microscopy, neural recordings, and when it comes to how we influence it, genetic manipulation.
So one aspect is to understand what the system is doing – the readout. The other is about influencing the system via gene manipulation technologies, which end up becoming gene therapy in the end. The same type of gene vectors that we use in the lab for doing experiments will end up a therapeutic setting.
How would you describe gene therapy to someone who is not familiar with it?
The retina is a biological computer that has many different kinds of elements, which we call cell types. Let’s say the retina has 100 different cell types, and we’d like to target a specific cell type for repair. The task for gene therapy is to create a little tool that we can inject into the eye, which would enter many different cells, and somehow it figures out which cell it is in and makes a therapeutic protein.
What we use are called gene therapy vectors. You can imagine we inject into the eye 100 billion little balls. Each ball has DNA in it, and the DNA has two parts. One is coding for the gene you want to express and the other is a postal address that determines in which cell type the gene will be expressed. In the end, it’s a very simple device.
What have been your key findings so far?
I would start with a key finding from when I was a graduate student. We discovered that the retina is not only a simple camera that detects a single image, but it performs a number of “Photoshop” like operations. Let’s say you look at a face and your retina performs a kind of operation that detects a particular feature of the face. For example, it detects the edges on the face. But the retina not only performs a single “Photoshop” operation that detects the edges but dozens of different operations. At the end the retina describes the face to the brain by showing a bunch of different images about the face. That is one of my first discoveries.
Later on, we worked to understand how these different face representations – these Photoshop operations – are computed in the retina. For example, we discovered a retinal circuitry that detects approaching motion. Later, we were interested in how specific types of retinal cells connect to the brain. Borne out of these studies were new therapeutic avenues to try to repair problems that occur in the visual circuitry.
What is your outlook on the remaining pieces of the puzzle?
There are a lot of remaining pieces! We can say that most of the pieces are remaining. But in at least a few things we are stepping forward. Medicine is very slow. Understanding is a bit faster, but with each understanding, there are more questions that arise. Understanding the visual system is a daunting task and I think we go step by step.

About Dr Botond Roska
Botond Roska obtained his M.D. at the Semmelweis Medical School, a Ph.D. in neurobiology from the University of California, Berkeley and studied genetics and virology as a Harvard Society Fellow at Harvard University and the Harvard Medical School. He then led a research group at the Friedrich Miescher Institute in Basel from 2005-2018. In 2010 he became Professor at the Medical Faculty and in 2019 Professor at the Science Faculty of the University of Basel. Since 2018 he is a founding director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB). At IOB he leads a research group focusing on the understanding of vision and its diseases and the development of gene therapies to restore vision.