
Exploring how neural activity shapes brain development
An interview with Dr Michael Stryker, Professor and W.F. Ganong Chair of Physiology at UCSF, conducted by April Cashin-Garbutt
What if the adult brain could regain the plasticity of early childhood? Following a recent SWC seminar on competition in brain development, Dr Michael Stryker explains how his lab has been studying the reactivation of critical periods in brain development and the potential for this to open new avenues for therapeutic intervention.
In this wide-ranging conversation, he reflects on his journey through neuroscience, including his time as a postdoc in Hubel and Wiesel’s lab (which almost didn’t happen), and the surprising discoveries that continue to shape his research today.
What first sparked your interest in neuroscience, and more specifically, in understanding the visual system?
It’s hard to pinpoint exactly, but I think it began during my time studying philosophy. Vision is such a dominant metaphor in how we talk about perception and understanding. For example, terms like “enlightenment” or “insight” are all visual.
But my real excitement for neuroscience came from working in Jim Olds’ lab as an undergraduate, studying the brain’s reward systems. Later when I was at graduate school, I joined a lab focused on eye movements and vision. I started learning a lot about the visual system and how it was one of many interesting things that the brain does. From there, I never looked back.
How did your postdoctoral research with Hubel and Wiesel influence your scientific journey?
I was initially unsure about whether to do a postdoc, as my PhD work had already attracted a lot of attention, and I’d been offered faculty positions without doing one – something that was unusual but not unprecedented in those days.
But as I was finishing grad school in Boston at MIT, I was invited to give a talk at Harvard in Hubel and Wiesel’s department. The discoveries during my PhD research, obtained using then-new laboratory computers to measure neuronal responses, showed that Hubel and Wiesel were correct about several things that Horace Barlow’s protégés Colin Blakemore and Jack Pettigrew had got wrong. After my talk, I spoke with Hubel and Wiesel, and they invited me to join them. It turned out to be one of the best decisions I ever made!
Harvard Neurobiology at the time was a unique environment. It was the first neurobiology department in the world where biochemistry, cellular neurophysiology, neuroanatomy, and systems neuroscience were brought together in a single department. We lived and breathed science. I learned more in those years than I ever imagined, especially from teaching and being taught by fellow postdocs in different areas such as molecular biology.
It was a wonderful time, and I had the opportunity to work with terrific colleagues including Bill Harris with whom I shared an office. Bill and I did experiments together, and also spent months debating the most important questions in neuroscience. We eventually agreed that understanding how neural activity shapes the development of the nervous system was key, and that question guided the next decade of our independent careers. I feel very lucky that I did the postdoc.
Your lab has been instrumental in establishing the study of the visual cortex in mice. What kinds of questions do you think mouse models can help us solve?
Despite their limitations, mice are incredibly powerful for studying the mechanisms of neural development. The genetic tools available in mice have allowed us to make enormous progress. At the Allen Institute, where I serve on the board, researchers have shown that many cell types in the mouse cortex have direct analogues in humans. That said, there are limits and ultimately, we need to bridge the gap between mice and humans, especially if we want to develop therapies.
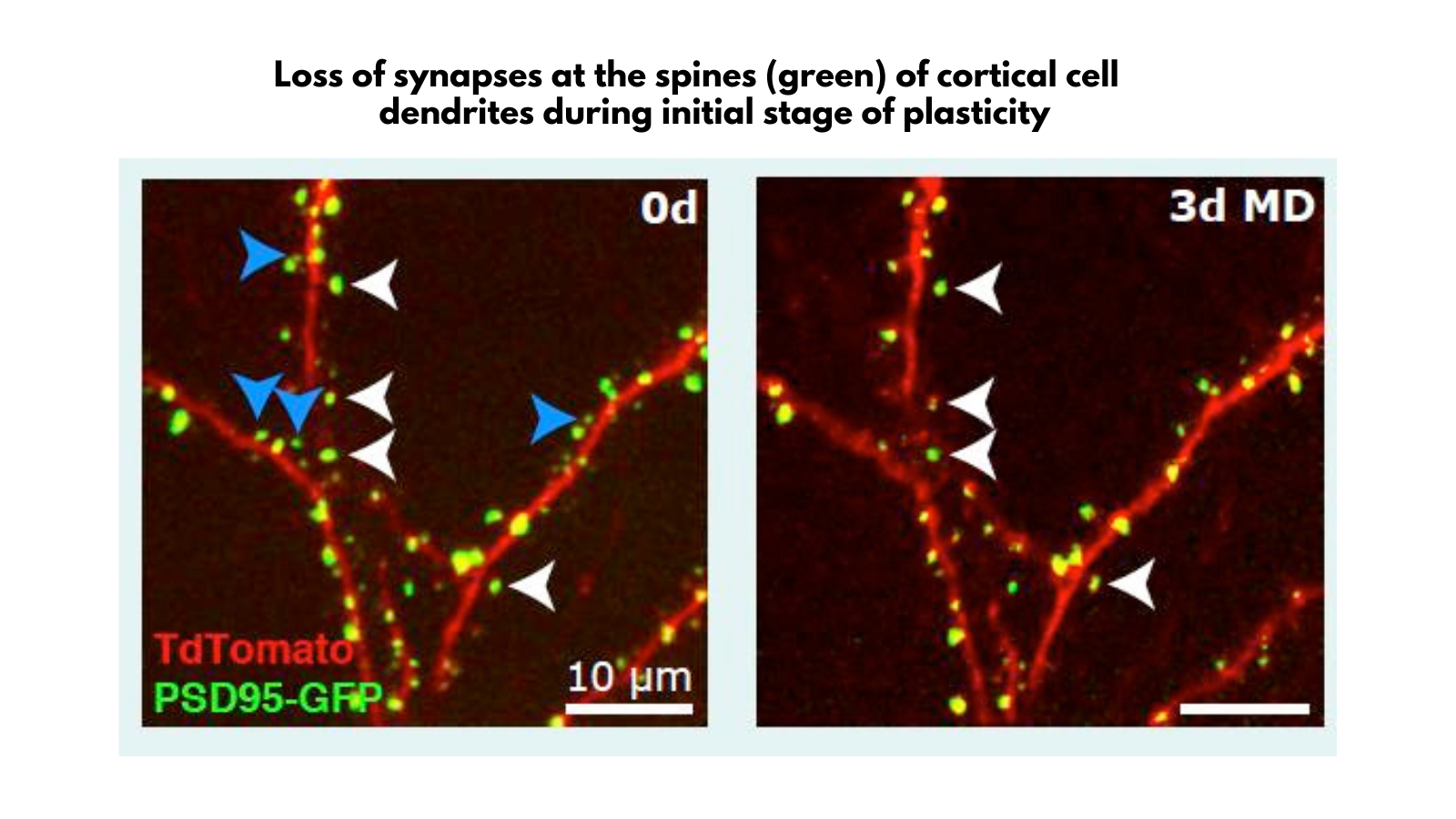
How much is known about the difference between the limited plasticity in the adult brain and the much greater plasticity during critical periods in early life?
That’s a really interesting topic. Some mechanisms of plasticity are unique to early life, while others persist into adulthood.
One of the most exciting discoveries we made with colleagues at UCSF over ten years ago was that the critical period in the development of the visual cortex in mice, which is about 10 days of sensitivity to monocular deprivation, is critically influenced by inhibitory neurons.
This gave rise to a collaboration between my lab and Arturo Alvarez-Buylla’s lab, which focused on stem cells. Postdoctoral fellows in our labs, Derek Southwell and Sunil Gandhi, showed that transplanting embryonic inhibitory interneurons into the adult cortex can induce a second critical period – essentially recreating juvenile-like plasticity.
We’re still trying to understand the exact mechanisms involved, but the therapeutic implications for recovery after neuronal injury could be profound. So far, we know that it is only two particular kinds of inhibitory interneurons that can do this if they’re transplanted. We know that they have to release their main neurotransmitter to create the second critical period. And we know that they don’t just create a circuit of their own, but that the donor embryonic neurons induce changes in the host circuitry.
While we can achieve this effect by transplanting neurons themselves, we have yet to identify a drug or other treatment that can do this. Together with our colleague Andrea Hasenstaub, Arturo and I are now pursuing a couple of other approaches to try to figure out the mechanism.
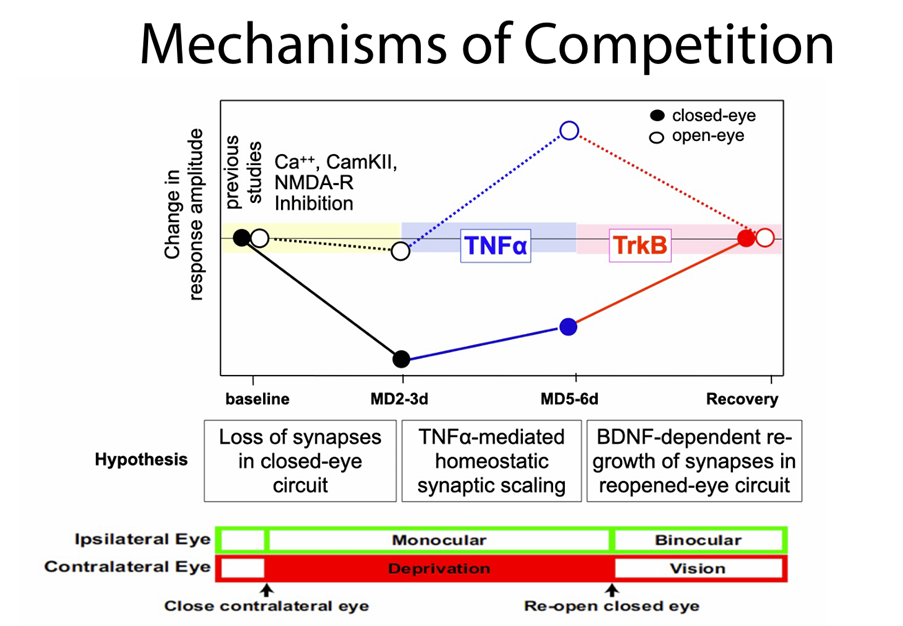
Your research on competition in brain development revealed that competition can arise from the interaction of distinct mechanisms that are not inherently competitive. Were you surprised by this?
Yes, very. A prevailing idea was that neurons compete for neurotrophins, but our work in mice showed that the outcome of competition can emerge from the interaction of distinct, non-competitive processes.
It’s a bit like a sports team where players aren’t directly competing but still produce a competitive result. For example, athletes competing in a decathlon or heptathlon take part in multiple events. Their performances in different disciplines are aggregated together into a final score. Each event is a separate process and not always head-to-head, but the overall result is a competition.
These findings challenge our assumptions about how developmental changes occur and remind us that biology often defies our simplest explanations.
Can you please explain how you devised a novel approach to reveal the neural code at different levels of the mouse visual system? How can the neural encoding manifolds you’ve created be used to probe machine learning networks?
We wanted to understand how mice actually see, especially since they perform visual tasks that earlier studies suggested they shouldn’t be able to do, and we wanted to understand natural vision computationally.
Working with my colleague Steve Zucker, we developed the idea of an encoding manifold – a way to organise neurons based on their responses, rather than focusing on the stimuli. Zucker’s and my subsequent work together with retinal neurobiologist Greg Field revealed a profound difference between the ways that visual neurons are organised in retina and cortex. All our work on this problem depended on talented and creative postdoctoral fellows, most prominently Luciano Dyballa, who now has a faculty position in Madrid.
This approach allows us to visualise the structure of neural responses in a way that’s grounded in the data. It’s not just about pretty pictures; the distances on the manifold reflect real differences in neuronal properties. While we can’t yet infer the circuit from the manifold alone, as different circuits could produce the same manifold, it’s a powerful tool for probing both biological and artificial neural networks.
Your recent work has demonstrated multimodal signals even in primary sensory cortical areas. Do you think there are limits to this multimodal potential?
Yes, I think there are limits. Our experiments are designed to show significant effects. But just because something is statistically significant doesn’t mean it necessarily matters to the animal. I am confident that our demonstration of a high-gain state in the visual cortex is real and operates in naïve animals. But in highly trained animals, the entire brain can become engaged in a task.
Ultimately, I think the future lies in studying natural behaviour in freely moving animals. That’s where we’ll get the most accurate picture of how the brain really works.

About Michael Stryker
Michael Stryker studied at Deep Springs College and the University of Michigan, where he earned a B.A. in philosophy with a minor in mathematics and worked in the laboratory of James Olds. He earned the Ph.D. in Peter Schiller's laboratory at M.I.T. in 1975, followed by postdoctoral research with David Hubel and Torsten Wiesel at the Harvard Medical School. He joined the Physiology Department and nascent neuroscience program at UCSF as an assistant professor in 1978, holds the W.F. Ganong Chair of Physiology at UCSF, and serves on the Board of Directors of the Allen Institute. He has been honoured by the W. Alden Spencer Prize from Columbia, the Ralph W. Gerard Prize from the Society for Neuroscience, and by election to the American Academy of Arts and Sciences and the U.S. National Academy of Sciences.
His laboratory’s research focuses on the role of neural activity in the development and plasticity of precise connections within the central nervous system. Most of his work has been on the visual system, in recent years on the visual cortex of the mouse. Current experiments seek to understand the cellular and neural circuit mechanisms of activity-dependent cortical plasticity, the interactions between neural activity and molecular cues in the formation of cortical maps, the difference between the limited plasticity in the adult brain and the much greater plasticity during critical periods in early life, and novel mathematical means for understanding cortical coding. His experiments take advantage of transgenic mice and optical as well as electrical approaches for recording from and labelling and perturbing connections of specific cells.


