How single neurons broadcast novelty through the brain
To function well in an unpredictable world, the brain needs to detect and respond to unexpected events. But in the cortex, individual neurons are only weakly connected, and each one is already bombarded by thousands of signals every second. So, it’s not clear how a single new spike, representing a novel event, can have much effect on the broader brain network.
Dr Dietmar Plenz, from the National Institute of Mental Health, recently spoke at SWC. He described his work that shows how even a single artificial spike in a cortical neuron can trigger widespread and meaningful responses across the surrounding network in awake mice. Despite the sparse and weak connections, these signals spread efficiently, even within the noisy and highly active environment of the brain. The key to this may be that the cortex operates in a critical state - a dynamic balance between order and chaos - allowing small events to have large effects.
His work has found that single novel action potentials scale in a predictable way, consistent with theoretical models of critical brain dynamics, and can be decoded to identify where the spike originated. Surprisingly, this communication does not require tightly synchronised firing or bursts of activity, challenging traditional models of how information spreads in the brain. In this Q&A, Dietmar discusses some of his work and its implications.
What first drew you to neuroscience?
I became a neuroscientist because I was originally very interested in complex systems. I felt that our world is composed of complex systems where many, many agents interact.
Those interactions are very unpredictable, but sort of stable. My original intention was to study ecology and ask what stable ecosystems are, but two things happened. One was the recognition that it's really hard to collect all the data to study an ecosystem - that would take a long time. The other was that in the 1990s, the idea of a stable ecosystem was probably wrong.
So I studied neuroscience. In brain research, the conceptual problem is the same. And, you can collect much more data in a shorter time.
What are the key questions you're aiming to address now with your research?
I think we don't know what computation in the brain is. We just see a lot of neural activity. We see that even the simple steps in computation involve many, many neurons and many, many areas in the brain. And we don't know why. We can guess, but essentially, we don't have any algorithm that tells us what the computation was. And I think once we find this, we will progress a lot. This would include all the implications with respect to fixing diseases as well as manipulating brain states.
Why is it important to understand how just one single action potential spreads?
It is the quest for the essential unit in the brain. It is staring us in the face – it is an action potential. That is the computational equivalent of a brain bit.
Once it is produced, the cell spends a lot of energy to make sure it propagates properly down the axon and reaches the 20,000 neighbours it's supposed to reach. The brain wouldn't pay attention to that if it wasn't important.
We have two parts in information processing - we have the dendritic side, the input side, where each nerve cell has to deal with some 20,000 inputs per second. Then, it makes a decision. I'm going to fire an action potential, and then it makes sure that this action potential reaches its 20,000 targets.
So I think this is the core unit of computation. We haven't addressed the distribution aspect of that piece of information properly, and that's what my work is about.
You've challenged the idea that synchronised or burst firing is essential for information transmission. What do your findings suggest about how the brain might be encoding or responding to novelty?
That's a good question, because the outcome could have been very different. It could have been that if we induce only one action potential, the network doesn't listen. But if we induce two or three or four action potentials within 100 milliseconds, then the network listens. You would have expected this, and not a straight line.
But we have confirmation that the single action potential counts. There's no qualitative difference between a neuron making one action potential or making two or four or ten within 100 milliseconds. That challenges our ideas that synchronisation is important to have an impact.
It's like in a room of people at a party. If only one person shouts fire, do we listen? Or is it if two people shout fire, that's when we listen? What we are saying, based on these experiments, is that you only need one person.
It doesn't mean that synchronisation doesn’t still play a role. We have ongoing experiments where we stimulate two cells together, and we want to know whether the outcome is additive or whether something else happens on top of this.
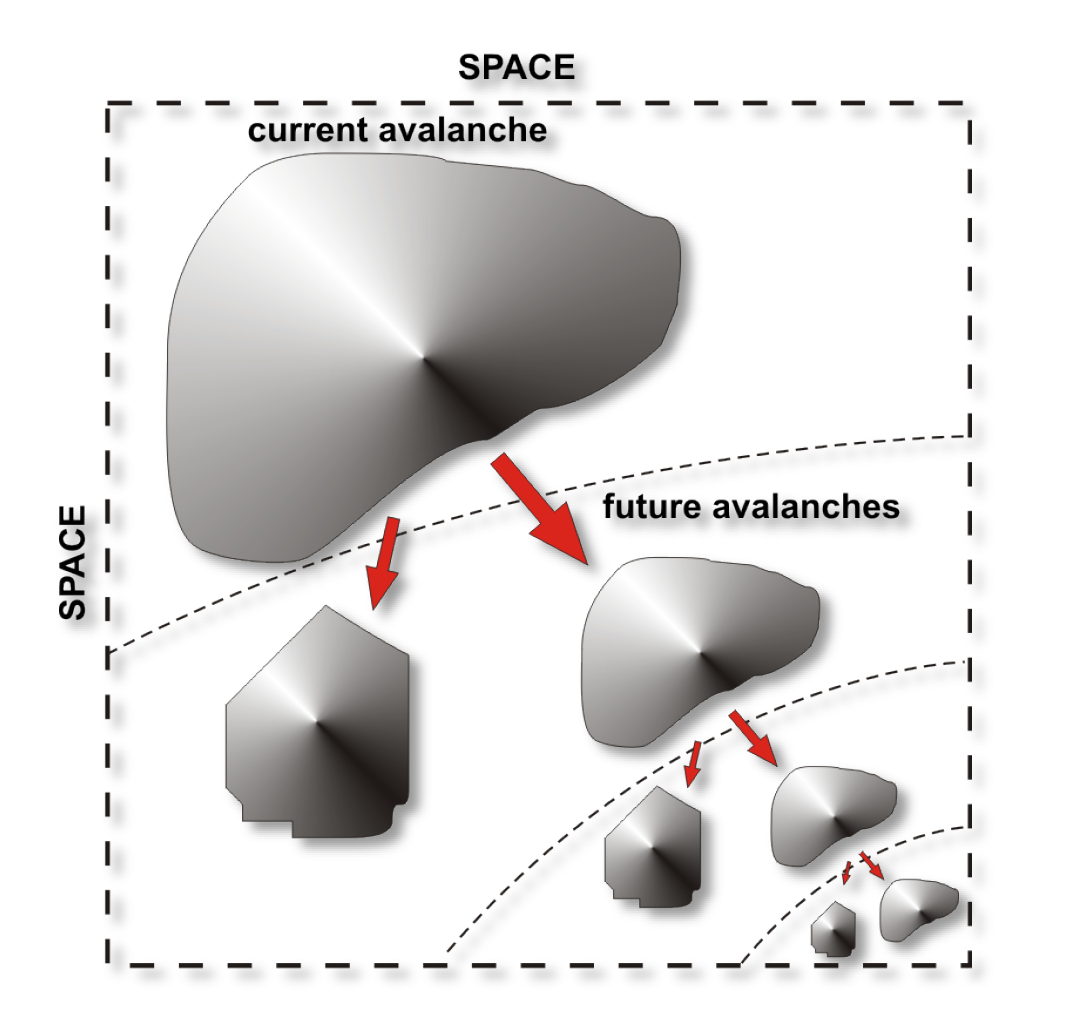
Could you explain criticality, and what it means for the brain to be in a critical state?
It's a statement about how excitable the network is. And how well it can transmit information that has entered the network locally to other sites in the network.
If that excitability is low, then information that enters somewhere in the network probably stays in that area and doesn’t travel. But if the excitability is higher, this information can propagate further. If there is too much excitability, then any local information would make the network go haywire, because these are all excitatory neurons and they excite each other. At that point, when all local information engages the network into a major response, that would be seizure activity.
So criticality is then the answer to solving that problem that you don't want to just stay local, because then you can't communicate with other sites in the network, and you can't be too excitable because then you take over. The critical state gives you exactly that optimisation.
Could you describe the potential implications of your findings? Would they help us understand some psychiatric conditions?
The framework of criticality gives you numbers. Real absolute numbers, like the number that I talked about in my seminar was 0.285 - it's a crazy number. Where does that number come from? Why would we think to label something as complex as the human brain with just a number? But these numbers describe critical networks that we have found experimentally. And because these are absolute numbers, we can simply measure brain activity, and if you don't meet that number, we can tell that something is off. We don't even need a control group.
So these are very absolute biomarkers of normal brain function. And then we can show that these numbers change.
In animal models of disease, for example we have a schizophrenia animal model, these numbers change. Corticosterone administration in animal models can induce anhedonia, a core symptom of depression characterised by a loss of interest in previously enjoyed activities. In this animal model, the numbers change. But when the animals recover, those numbers come back.
So it gives us a real mathematical framework for optimal brain functioning.
Where do you want to take your research next – what is the next piece of the puzzle?
We are still working on these very, very fundamental principles of how individual cells, either alone or in cooperation, send information into the neighbourhood. I think these are very basic principles that will give us insights independent of whatever behavioural paradigm you're doing.
The typical experimental approach is that you pick your brain area, you pick the quality of that. For example, a brain area that deals with auditory information. Then you make the animal recognise something within that paradigm. You ask what the connection is between information in and behaviour out.
But we are saying, actually, let's just see what the principles of cortical networks are, in awake animals, and independent brain areas.
I expect to find what we’ve already shown in the frontal cortex to also be found in the motor cortex – that is one of the experiments we are running now. If that's correct, then we are looking at something that is fundamental. It is action potential generation in a neuron and how that spreads in a network.

Biography
Dr. Dietmar Plenz is Chief of the Section on Critical Brain Dynamics at the National Institute of Mental Health (NIMH), USA. He studied at the Universities of Mainz and Tübingen, earning his Ph.D. in 1993 under Prof. Valentino Braitenberg and Ad Aertsen at the Max Planck Institute of Biological Cybernetics. Joining NIMH in 1999 as a Tenure-track Investigator, he gained tenure in 2006. Dr. Plenz’s discovery of neuronal avalanches established the field of critical brain dynamics. His lab integrates electrophysiology, imaging, and neural modeling to explore optimization in cortical information processing in rodents, non-human primates, and in vitro systems.