Probing the role of dopamine in motivational states
An interview with Professor Neir Eshel, Stanford University, conducted by Hyewon Kim
How can an economics concept about consumption and cost be applied to understanding motivational states in the brain? In a recent SWC Seminar, Professor Neir Eshel shared his research on how dopamine release in the reward system of the brain depends on varying levels of cost and benefits in a given situation. In this Q&A, he expands on applying his lab’s key findings to understanding motivational states in individuals, how his dual research and clinical background informs the direction of his work, and more.
What first sparked your interest in how dopamine is involved in cost-benefit calculations in behaviour?
I have been focussed on dopamine and learning through reward-prediction errors for a while. I kept running across older literature that wasn’t as much about learning as it was about performance, vigour, and cost-benefit calculations. Those experiments struck me as important, shedding light on the patients I see in the clinic who vary so much in their level of motivation and the way in which they seek out rewards.
I wasn’t sure if learning could explain everything. So, what I wanted to do was to take some of the newer tools that we have to record and manipulate dopamine, and try to map them onto these older concepts about cost-benefit calculations or motivation. These concepts are more about motivational states that can vary from individual to individual or day to day and predispose one to becoming addicted, for example. Over time and naturally, I fell into this idea to see if we can come up with a slightly different way to map dopamine onto these constructs.
Does your background in completing a residency in psychiatry inform your decisions about experiments and analyses?
I try to choose scientific problems that seem relevant to the patients I see. When I interpret the results of my experiments, I’m always thinking in a part of my mind, ‘Does this make sense from a clinical standpoint? Does this tell us something that relates to the patients’ symptoms or the experiences they tell me about?’ It’s an iterative process and I don’t use my neuroscience in every clinic visit or use my clinical experience in the lab every day, but at a more holistic level, I do try to relate the two.
One of the ways I’m doing that is by seeing in what clinical context these concepts of mapping dopamine onto motivation might play a role. One of them is in addiction. We’re administering different drugs in this model and trying to see if we can predict, for example, which individual animal is more likely to become more addicted to a given substance, or which substance is more likely to have a bigger addictive potential than which other substances, all based on the dopamine firing patterns that we can record in tasks like this. I’m thinking about ways we can use this as a screening tool – to screen treatments for addiction, for example.
I can also ask, if I try to look at anhedonia models in mice, can this behavioural paradigm or pipeline tell us something about what happens to different individuals in the context of chronic stress? Not every individual will respond in the same way, but maybe we can predict or track their response to stress based on their dopamine or performance on this task.
How much is known about the way the dopamine system in the brain encodes motivation and reward? What about cost and benefit?
I think the dopamine field is overwhelming and I never feel like I’ve caught up even after years of trying to read the literature. There are always papers that I miss and there are people whose work is very inspiring that I’ve only just come across. It’s daunting to study this field. I even tried several times to move away from it because it’s so daunting, then I keep on getting drawn back because it’s so interesting and dopamine seems so powerful in terms of driving behaviour.
A lot has been done on every conceivable aspect of dopamine and reward-seeking. I’m trying to do my best to know what’s been done, and not just replicate, but try to extend it. Partly, it’s about using new tools. Even if the question was similar 30 years ago, the tools that we have now are different. Hopefully they’re more precise and able to both record and manipulate dopamine in a more temporally and spatially precise way. So we’re trying to take advantage of these new tools, and shed new light on questions that had been asked and partially answered many times over decades.
It's also about bringing new perspectives on demand curve analysis, which had been used in addiction, but as far as I know used very rarely in any other type of models for neuropsychiatric disease. Maybe it will be relevant for something like stress responses. As far as I know, people haven’t spent a lot of time working on that, and I think it’s reasonable to get started.
Why do you use the concepts of consumption and cost from economics literature?
It seems to me a type of analysis that is fairly easy to understand and is highly robust across species translationally. It allows me to dissociate two parameters that could each be important and relevant in various conditions: First, how much of a given reward would you want if cost weren’t an issue – basically if it were given to you for free? Second, how much work are you willing to put in to maintain that preferred level of consumption?
I can see both consumption and cost sometimes co-varying, and sometimes independent from one another. So being able map them onto the dopamine circuitry seemed like a potentially illuminating approach. This is especially the case in addiction where demand curves have been used for a long time to determine how addicted someone is, or how willing they are to undergo punishments or severe costs and still maintain their use.
So, if we can probe this cardinal feature of addiction, this motivation to continue consuming the drug despite all the adverse consequences – if we can figure out what’s happening in the brain around those choices, I think we can make a difference.
What have been your key findings so far?
One finding is that it appears there is a relationship between how much work you put into getting a given reward and how much dopamine is released in response to that reward. For example, for the same reward, if you worked really hard for it, more dopamine is released than if you worked less hard for it. I think that is related to this concept of sunk cost, which is an interesting phenomenon that people have known for a long time, and states that we value things more if we work hard for them. Maybe this finding is a neural representation of that psychological concept.
The other interesting finding is that in high motivational states, when the animal is more willing to work hard for a given reward, compared to lower motivational states, in which the animal gives up more rapidly, I see a nice mapping of that onto dopamine release. But it happens in the opposite way that I expected. In high motivational states, there seems to be lower rapid dopamine release in response to rewards. And we’re still working on what that could mean and what mechanisms might come into play, or how that happens.
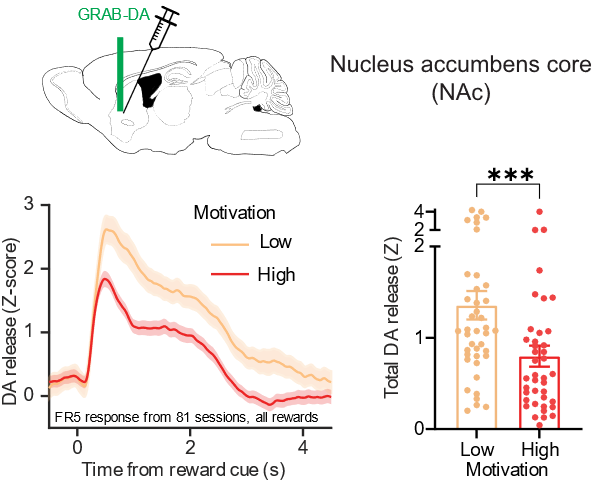
Reward-evoked dopamine release inversely reflects motivational state.
This finding is surprising and interesting, although it does relate to a long-standing finding in the addiction literature, which is that stimulant users who are more likely to relapse or who are more seriously addicted to the stimulants have been shown to have lower dopamine release. This was found with the tools available to measure dopamine release in people, which are less temporally precise than the ones we can have in mice, but have similar results.
There has been a long-standing debate in the field, as this finding can be confusing for a lot of people, who just think more dopamine is always going to be more motivating or better in some way. But it looks like it’s more complicated than that. You do need some level of dopamine to motivate behaviour, but it looks like there is an inverse relationship between the motivational state and how much dopamine is released for each reward. We’re still working to understand that, but it’s an interesting and provocative finding in itself.
What experimental techniques did you use to find this out?
We trained mice to poke their noses in a specific hole a certain number of times to gain access to a reward and we used either a sucrose reward for mice that were hungry and liked the sugar water, or sometimes we actually used a technique called optogenetics to elicit dopamine release directly. In the case, the dopamine release was the reward that they were working for. We trained mice on this task and varied the amount that they had to work for a given reward, and based on that we could draw these demand curves and extract the parameters of interest that I mentioned before.
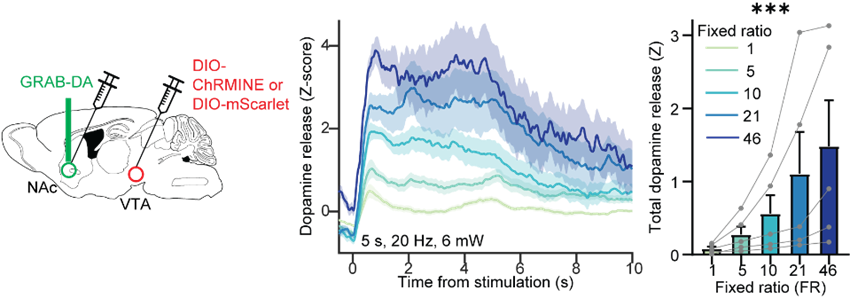
Working harder for the same optogenetic stimulation of dopamine axon terminals in the nucleus accumbens results in increased dopamine release.
At the same time, we could record dopamine release second-by-second using a technique called fibre photometry and this genetically encoded dopamine sensor called GRAB-DA. While mice are performing this task and poking different numbers of times to gain access to a reward, we are recording the dopamine release during the task in response to those rewards. That’s how we mapped all of these parameters onto dopamine experiments.
What are the main implications of your research?
I think it supports the view from the addiction literature that we might want to focus our energies on the low phasic dopamine responders as people who might be more liable to become compulsive users or are addicted to a given substance. If we could use this type of technique to screen individuals, determine what kinds of treatments might be more likely to work, or what interventions to do, that would be helpful.
We are currently using this to test a new drug for methamphetamine use disorder. We don’t have any treatments right now for people who are addicted to stimulants like methamphetamine, so I’m trying to see if this new drug that a collaborator developed might be effective for this disorder for rodents first and then for people if it works out. And if the mechanism involves the dopamine system, then we can use this technique to screen or enhance the efficacy of the drug, or choose the right version.
It may also have implications for our understanding of things like stress effects and anhedonia, or other types of symptoms that I consider disruptions or disorders of motivation. They include negative symptoms in schizophrenia or amotivation symptoms in people with dementia. These are examples in which there is a clear disruption to motivational circuitry, and if we have a better understanding of what that circuitry is, then maybe we can better target treatments.
What’s the next piece of the puzzle that you’re excited to work on?
There are many that I am actively working on or thinking about working on in the near future. I’m really interested in what happens downstream of dopamine – we can map variations of dopamine release at different times or motivational states, but what does that mean to neurons that are receiving the dopamine signal? Looking at different genetically defined populations of neurons that are receiving dopamine input and trying to see how they use that dopamine input to ultimately motivate behaviour is a really interesting question that we’re trying to get at now.

Another thing we’re doing is trying to figure out the role of dopamine in response to chronic stress or various stressors. With a translational eye, can we predict who is likely to respond in a certain way to chronic stress, and can we track how they respond to different stress levels? I think that is a fascinating future direction.
Also from the methodological standpoint, I’m super curious about how is it that when we use optogenetics tools, most people assume that whenever you use it, you get more or less the same results. But what we find is that there is a lot of context dependence to what happens to neural activity, or in this case dopamine release, in response to the same pulse of optogenetic stimulation. We’re trying to figure out a mechanism that could explain that.
Another piece of the puzzle has to do with local control of dopamine release – there is a lot of controversy now over how much there might be distinctions between what is happening at the level of the cell bodies of dopamine neurons, and what’s happening at the axon terminals and where dopamine is released locally. So maybe I now have a platform to start to get at that question.

About Neir Eshel
Neir Eshel joined the faculty at Stanford University in 2021 as an assistant professor in the Department of Psychiatry & Behavioural Sciences. He received his A.B. from Princeton University, M.Sc. from University College London, and M.D./Ph.D. from Harvard University. For his Ph.D. work with Naoshige Uchida, he used optogenetics, electrophysiology and behavioural approaches to probe the neural circuit regulating dopamine release as mice learned about rewards. He then completed psychiatry residency at Stanford University, where he extended his interest in dopamine to study the role of this circuit in clinically relevant behaviours, including aggression and addiction. In addition to running a small lab (www.staarlab.com), Neir also maintains a clinical practice and hopes to translate his basic neuroscience findings into new treatment options for his patients.