Uncovering the neural basis of fear memories
An interview with Dr Sarah Melzer conducted by April Cashin-Garbutt
Following her SWC Virtual Seminar on neuromodulation of cortical circuits for fear memories, I caught up with Dr Sarah Melzer, Postdoctoral Fellow, Harvard University, to find out more about her research.
How do you define a fear memory?
Fear is when you feel something poses a threat or danger to your life or your wellbeing. It is not a general state, but usually something that is learned and associated with specific cues. In animal research we do experiments where we pair something that is originally harmless with an adverse event, so the animal becomes afraid of the harmless cue because it expects something harmful to happen afterwards.
What circumstances require flexibility in forming or recalling fear memories?
New fear formation and extinction are life-long processes. However, the requirement for flexibility becomes especially apparent in the context of diseases where we may wish to get rid of certain memories. For example, in post-traumatic stress disorder (PTSD), people that have experienced very distressing events, such as veterans that have been to war, can develop debilitating symptoms of increased anxiety, flashbacks and sleep problems as a consequence of their strong fear memories. Cues that posed life-threatening risks during a previous experience can then trigger flashbacks at a later stage. It is very important that fear memories are associated with very specific and relevant contexts. If the same stimuli appear out of context, it is crucial to know that the stimuli are safe in a different context.
Despite this need for specificity, lots of fear memories are important for survival and sometimes it is necessary to generalise these memories by learning one stimulus that is threatening and extrapolating to other stimuli that are similar that could also pose a threat to your life. For example, if children touch a hot oven once, they usually don’t do it a second time even if the oven looks different or is at a different place. We learn a lot of these generalisations as children, as the brain is most plastic then and can learn these associations more easily. But the brain is plastic in adulthood too, so we constantly learn new associations between adverse events, cues that serve as triggers and relevant contexts and understand what stimuli are beneficial or harmful for our own life and wellbeing.
If we understand how fear memories work in healthy brains, will that help us to understand disorders like PTSD?
The development of PTSD is often associated with pre-disposition of the person by genetic risks as well as by former life experiences. For example, adverse events in childhood increase the risk of developing PTSD.
While some basic processes of association learning are the same, there are specific processes that lead to the debilitating symptoms that follow later on and are not fully understood yet. Most people do not develop PTSD after experiencing adverse events, so when we conduct research it is important to use model systems that can replicate the pre-disposing factors that increase the risk of developing symptoms.
How much is currently known about the way the brain adapts to fearful experiences and how the activity of inhibitory neurons is regulated?
There are many levels of understanding how the brain adapts to fearful experiences. A lot of research has looked at the synaptic level to see how synapses are strengthened or weakened in certain pathways. Another level is to look at genes to see if they are upregulated or downregulated to change the expression of certain proteins when learning these associations between harmful and non-harmful stimuli. The next level is to look how the activity of neurons within relevant brain networks changes and how this is related to the changes on gene, protein and synaptic level.
Inhibitory neurons are generally important in regulating activity and plasticity in the brain. By either upregulating or downregulating the activity in the interneurons you can fine-tune the amount of plasticity that happens in relevant pathways. There is some literature, including my own, that shows the specific functions of inhibitory neurons in the auditory cortex and in the amygdala in regulating the plasticity and signal transmission to allow the information to pass through the local and long-range circuits and cause lasting changes in connectivity, gene expression and neuronal activity.
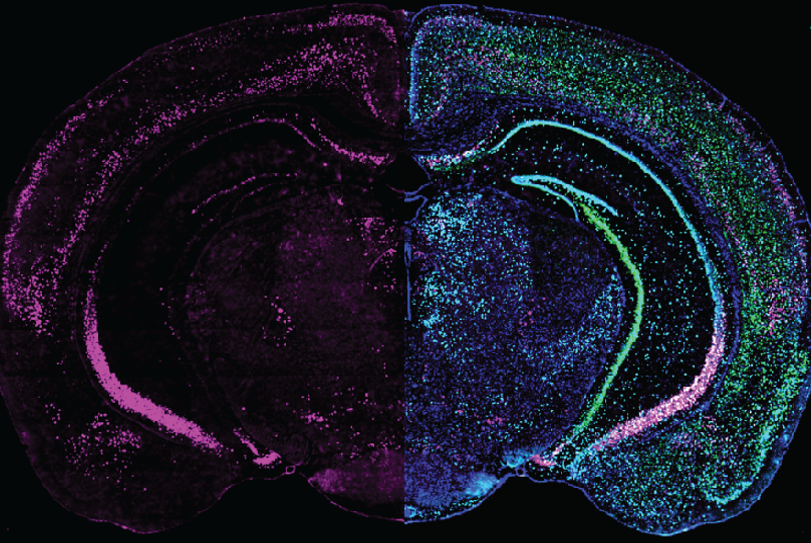
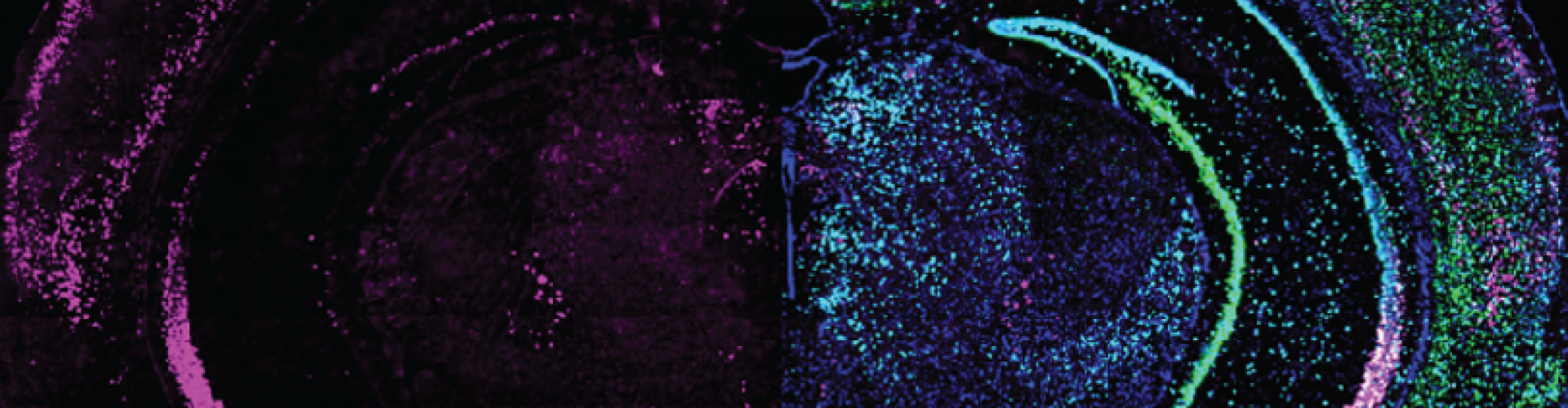
Expression of GRP (left half) combined with different cell markers (right half) in a brain slice
How did you further the understanding of neuromodulation of cortical circuits for fear memories?
There are hundreds of different neuropeptides in the brain whose functions and mechanisms of actions are largely unknown. My research looks at one specific neuropeptide – gastrin-releasing peptide (GRP). We figured out how GRP changes the activity and gene expression in one particular circuitry that is involved in fear memory. We are now developing ways to visualise the release of GRP so we can better understand what triggers its release and at what specific time points it becomes important.
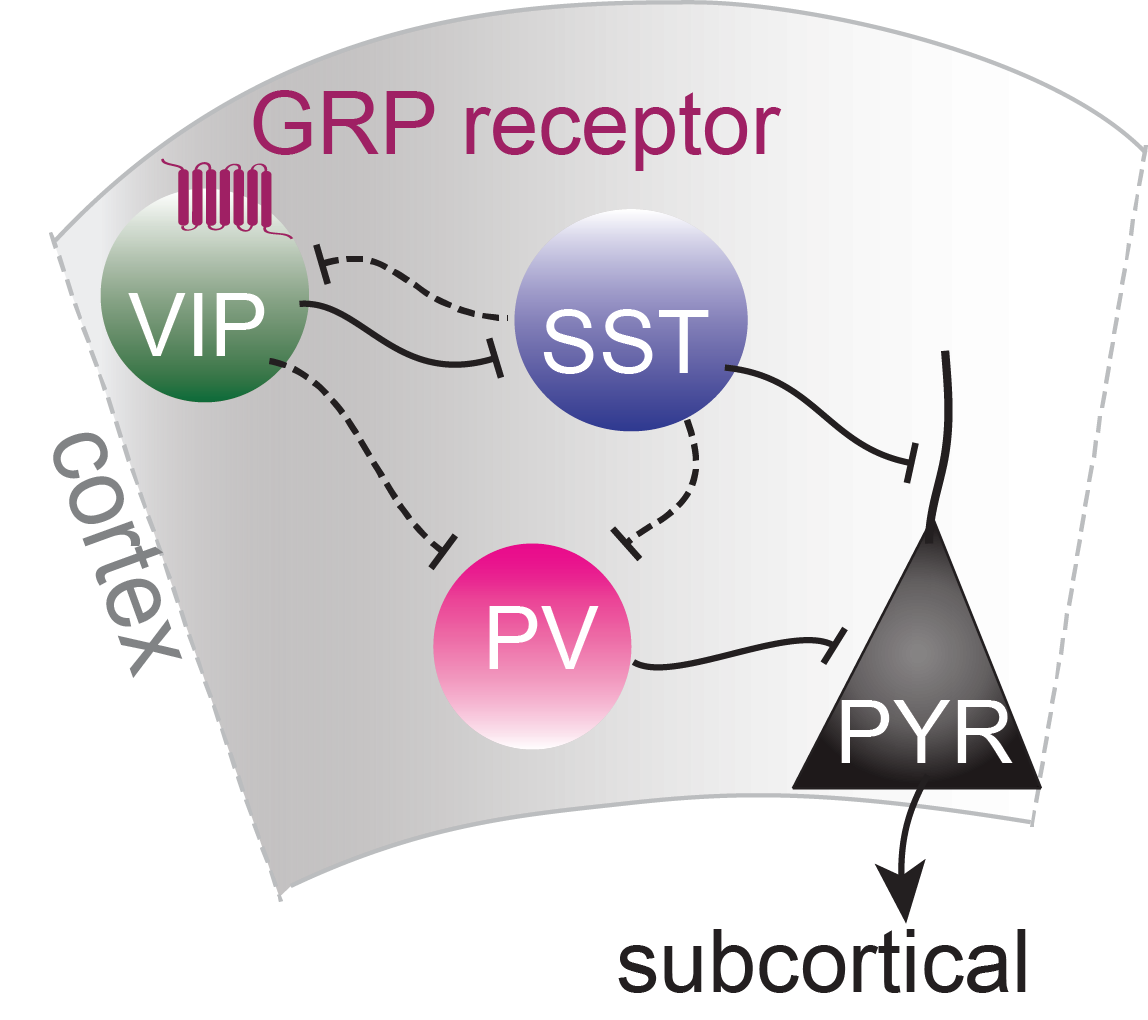
Schematic of cortical neuronal circuit with GRP receptor
Why did you choose to study GRP?
The main reason we studied GRP is because it was found to be expressed in one particular interneuron type called vasoactive intestinal peptide (VIP)-expressing cells that have been found to increase plasticity in the brain and might thus be a key to the flexible formation and extinction of fear memories.
If you look at the expression of all neuropeptide receptors, you find an extremely specific and individual expression pattern for every neuropeptide, such that there are hundreds of different communication channels that work in parallel in a context-dependent way. This allows very specific communication to different cell types in and across neural circuits.
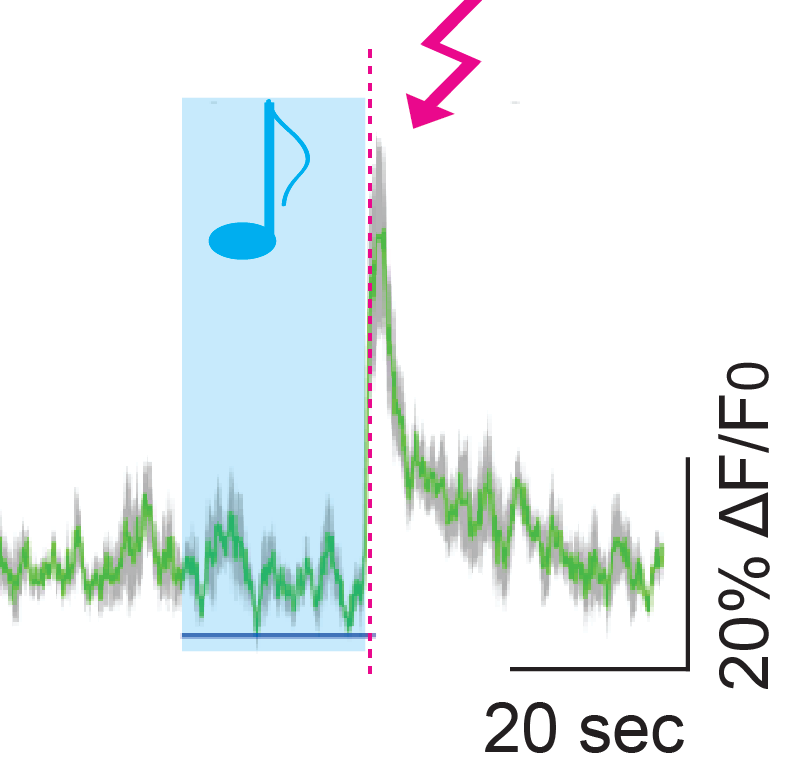
Response of some cortical VIP cells to sounds and shocks
Is the large number of neuropeptides and receptors going to make it more challenging to understand?
Absolutely! Understanding all this complexity is going to be challenging as there are around a hundred neuropeptides that all have their own specific way to affect the circuits and behaviours. In addition, every neuropeptide needs their own tool that needs to be developed, tested and verified for every single neuropeptide and receptor individually – this includes for example the development of CRISPR/Cas9 tools and sensors to visualise every neuropeptide to figure out how they are released and when they might become important in modulating circuit activity. This is going to be a lot of fascinating work for many researchers over the coming years!
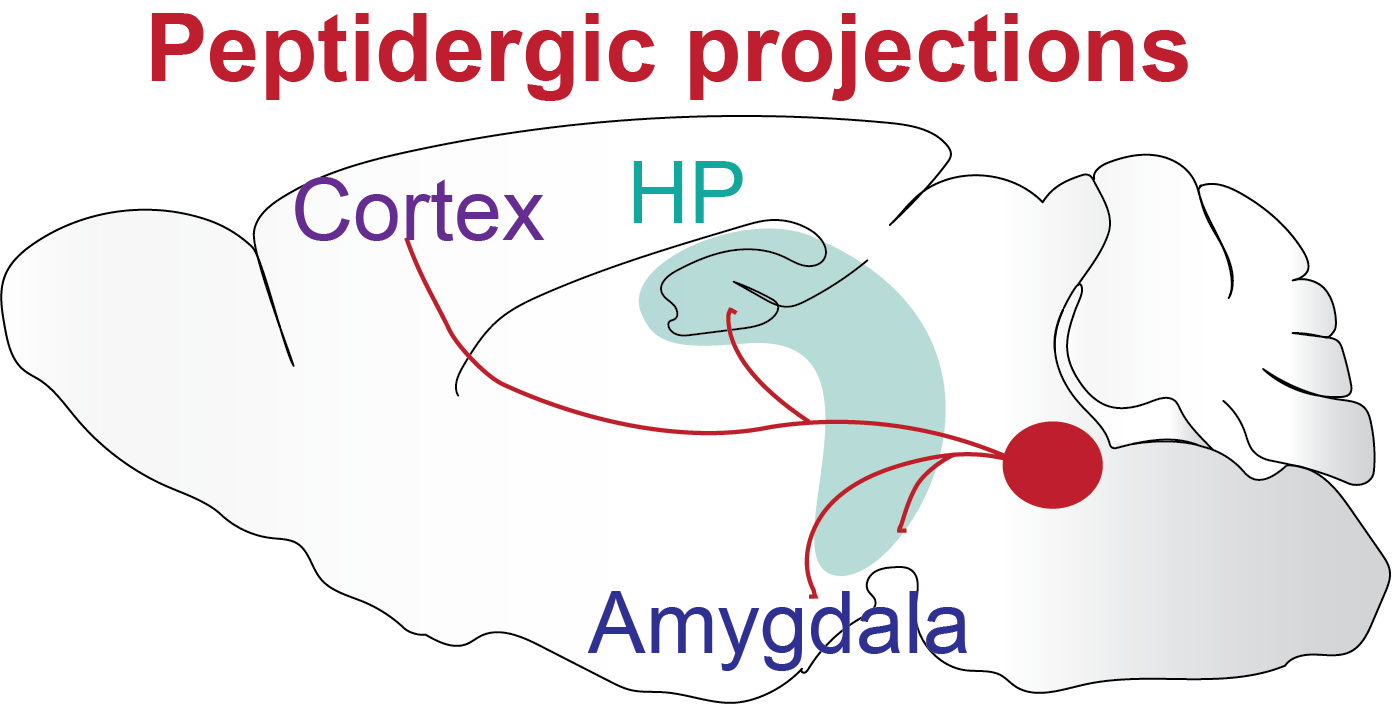
Schematic of a peptidergic control centre with brain-wide projections
What is the next piece of the puzzle your research is going to focus on?
Something I find fascinating is that a few neurons, located in very specific brain areas, express neuropeptides or other neuromodulators that can control the whole brain through brain-wide projections, which leads to brain-wide changes in circuit states and result in cognitive and emotional state changes. These changes in state affect the way we think, perceive, memorise, learn things, and make decisions on what to do next.
One example is our arousal state. Arousal helps us to quickly react to threats and learn new associations. On the other hand, excessive hyper-arousal is a common debilitating symptom in PTSD that causes sleep problems and increased anxiety. Another example is the emotional state that strongly influences our ability to learn. If you think back to your childhood, we memorise things that are associated with very positive or very negative emotions much more than anything neutral. I think a lot of this can be understood as brain state changes that are partly caused by these global brain-wide projection neurons that release neuromodulators and change how neuronal circuits react to environments and how they change the way we think, behave and respond.

About Dr Sarah Melzer
Sarah Melzer is a postdoctoral fellow in Bernardo Sabatini’s lab in the department of Neurobiology at Harvard Medical School in Boston. Her research focuses on the neuromodulatory regulation of functional GABAergic circuits in the mammalian cortex. She uses and develops novel approaches to study neuromodulator function on circuit and behavioural level.
Sarah received her Bachelor’s degree in Biosciences from University Muenster (Germany) and Rijksnuiversiteit Groningen (Netherlands), and a Master’s degree in Molecular Biosciences and Neurosciences at Heidelberg University (Germany) including research stays in Japan and UK. Her graduate research in Hannah Monyer’s lab at Heidelberg University focused on local and long-range cortical inhibitory neurons using in vitro and in vivo electrophysiological and optogenetic approaches. Her work was published in Science, Nature Neuroscience, Neuron and Cell Reports. She joined Bernardo Sabatini’s lab in 2015 and was supported by EMBO, DFG, Brooks and Gordon postdoctoral fellowships.
